Explain the Difference Between Iron Ii Nitrate and Iron Iii
The inherent acidity of the iron solutions all fell within the pH range of ca. IronIII profiles of flooded paddy soil incubated in the greenhouse indicated oxidation of ironII in the upper 6 mm soil layer.

How To Write The Formula For Iron Ii Nitrate Youtube
The appearance is just the same as in when you add sodium hydroxide solution.

. The reaction looks just the same as when you add sodium hydroxide solution. View 63 concept_reviewpdf from AA 1Name _ Class_Date_ Skills Worksheet Concept Review Section. Iron IK chloride is also dissolved in water and then sodium hydroxide solution is addedA reddish brown precipitate is obtained which confirms the presence of iron HI.
On the other hand changing ironIII ions to ironII ions is a reduction and therefore requires a reducing agent. Iron II nitrate contains an iron ion with a 2 charge and has the formula FeNO32. Aqueous solutions of iron III sulfate and lead II nitrate are mixed.
The Roman numeral shows that iron II nitrate contains Fe2 ions while iron III nitrate contains Fe3 ions. To list compounds useful in identifying the iron II and the iron III ions. However the extent to which FeII oxidation is biologically catalyzed remains unclear because no neutrophilic iron-oxidizing and nitrate reducing autotroph has been isolated to confirm the existence of an enzymatic pathway.
IronII nitrate FeN2O6 CID 9815404 - structure chemical names physical and chemical properties classification patents literature biological activities. If a reaction occurs in pan a of this question write balanced molecular equation for the reaction that occurs and include phase symbols. The brown ring test is test to positively test for the Nitrate ion.
The test follows sev. The precipitate again changes colour as the ironII hydroxide complex is oxidised by the air to ironIII hydroxide. It is predicted that a reaction would occur.
To learn how to confirm the presence of the iron III ion. The above image describes the structure of the Iron II III oxide. Fe 3 O 4 is the chemical formula of Iron II III oxide which has four oxygen atoms three iron atoms.
Explain the difference between ironII nitrate and ironIII nitrate. In Iron II Nitrate Iron has a 2 charge Fe2 and. Arrow_forward What mass in grams of solid ammonium sulfate would be required to prepare a 250 mL of 0925 molar solution of ammonium sulfate.
Compound Names and Formulas 1. Iron II chloride is dissolved in water and then sodium hydroxide is added. Therefore such bonds are called an Ionic bond.
To organize the data collected into a chart. Explain the difference between iron II nitrate and iron III nitrate. Measuring the pH of the FeII and FeIII salts separately demonstrated that the FeIII salts were responsible for at least 10-fold more inherent acidity 1 pH unit lower than the FeII salts.
Iron II III oxide structure Fe 3 O 4. Describe how covalent compounds are named. The compound will dissolve in molten stearic acid and decompose at about 120 C to give ironIII oxide-hydroxide FeOOH.
The overall reaction is the reduction of the nitrate ion by iron II which is oxidised to iron III and formation of a nitrosonium complex where nitric oxide is oxidised to NO. In the ironII case. When dissolved ironIII nitrate forms yellow solution due to hydrolysis.
Microbially driven nitrate-dependent iron Fe oxidation NDFO in subsurface environments has been intensively studied. FeCl 2 2NaOH FeOH 2 2NaCl. To design an experiment to determine if an unknown solution contains iron II or iron III ions.
Its basically a difference in the electron shell arrangement of the central Iron atom. Fe 2 O 3 is the chemical formula of IronIII oxide which has three oxygen atoms two iron atoms. The charge of the iron ions in each compound is different.
Iron II chloride is also named as. The charges are different shows the charge. An aqueous solution containing ironII ions Fe 2 is pale green in colour whereas that containing ironIII ions Fe 3 is yellowyellowish-brown brown in colour.
If you get a green precipitate the unknown substance is ironII. The Roman numeral shows that ironII nitrate contains Fe2 ions while ironIII nitrate contains Fe3 ions. In Iron II Nitrate Iron has a 2 charge Fe2 and.
Measurement of oxygen with a Clark-type microelectrode showed. The bond formation between iron and oxygen depends on the difference in electronegativity between them. In-situ iron isotope analysis of pyrites in 37Ga sedimentary protoliths from the Isua supracrustal belt southern West Greenland By Kazumi Yoshiya and Tsuyoshi Komiya Iron isotope fractionation during microbially stimulated FeII oxidation and FeIII precipitation.
Its basically a difference in the electron shell arrangement of the central Iron atom. IronII chloride and ironIII chloride are important inorganic compounds of the chemical element iron Fe. The charge of the iron ions in each compound is different.
IronFe is metal whereas oxygenO 2 is non-metal. Test for Iron II and Iron III Ions. How many grams of iron III nitrate is needed to make 250mL of 075 M solution.
A dirty green precipitate is obtained which confirms the presence of iron II chloride. To tell whether an unknown substance contains ironII nitrate or ironIII nitrate add a few drops of sodium hydroxide solution. What is the significance of the Roman numerals.
Changing ironII ions to ironIII ions is an oxidation and therefore requires an oxidising agent. The oxidation state of Fe 2 O 3 is 3. When heated to near boiling nitric acid will evaporate from the solution and all the iron will precipitate as ironIII oxide Fe 2 O 3.
A prefix is added to the first and second element. The key difference between iron II chloride and iron III chloride is that the Fe atom in ironII chloride chemical compound has a 2 oxidation state whereas the Fe atom in ironIII chloride compound has a 3 oxidation state. In the ironIII case.
The bond formation between oxygen and iron depends on the difference in electronegativity between these two atoms.
What Is The Formula For Iron Ii Nitrate Quora

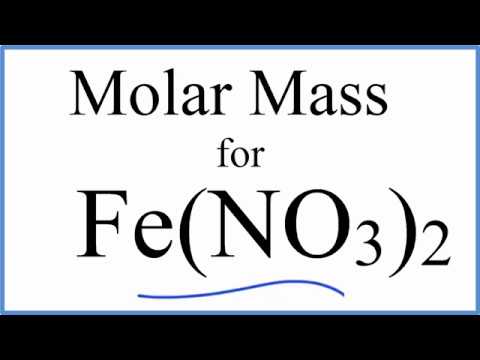
Comments
Post a Comment